Our Research Focus
Our research projects are organized into three Transition Topics – Resilience, Recovery, and Borders - that unite key pathomechanisms into more general disease concepts and appear particularly suited for clinical transition. These Transition Topics require larger and multidisciplinary teams of researchers including the integration of clinician-scientist expertise.
The research within these Transition Topics is structured as Tandem Projects.
The research is complemented by Technology Hubs that provide critical methodological support.
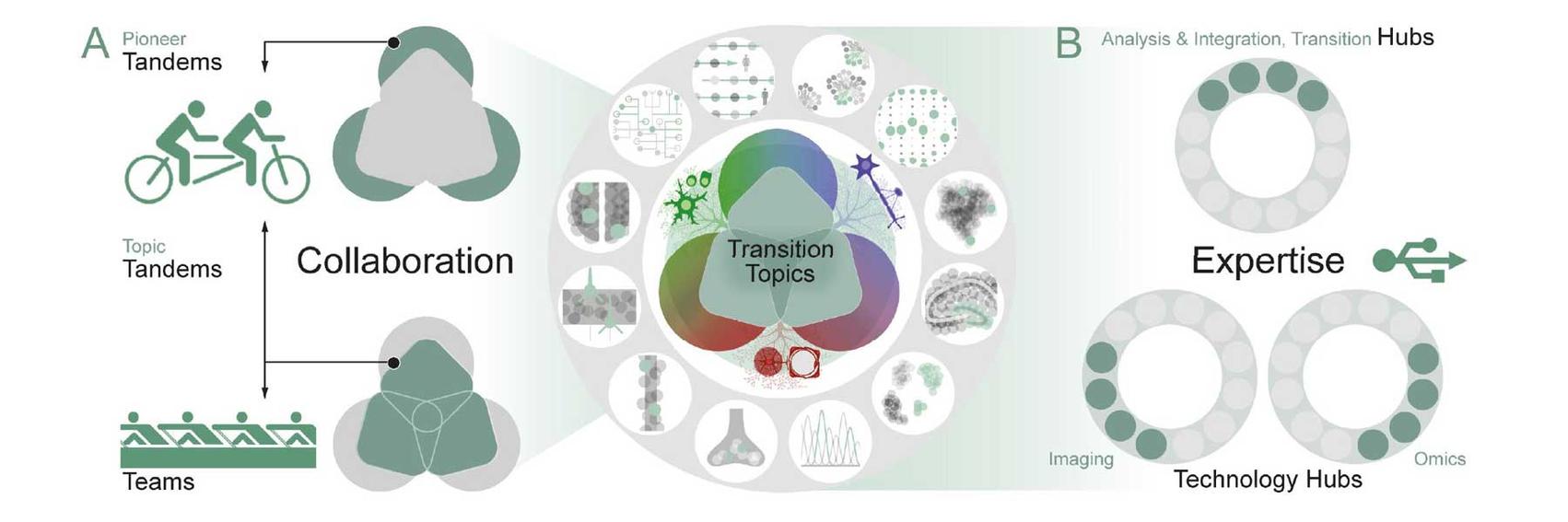
Structure of SyNergy’s research program: A. Tandems are the Cluster’s hallmark measure, initiated “bottom-up” by two SyNergy Investigators with complementary expertise (PIs, SyNergy Professors, or Early Excellence Academy members). Tandems either pioneer new scientific or technological terrain (Pioneer Tandems) or develop new aspects of the Transition Topics (Topic Tandems)—with the option of eventually initiating new or expanding existing Teams. The new Teams consist of four to six investigators from different disciplines, including clinical experts, and collaborate on one of currently three Transition Topics (dark green squares) to boost clinical transition. B. The Teams and Tandems are supported by Hubs and link to key infrastructure such as the Cluster’s Research Data Management Platform and its translational partners
Our three Transition Topics
Edbauer / Höglinger / Brendel / Haass / Korn / Zhou
Cell Resilience & Protection are vital processes for neurons. As postmitotic cells, neurons have limited regenerative capacity and—due to their long lifespan— readily accumulate age-associated damage to DNA, lipids, and proteins. Yet, neuronal loss tends to occur slowly and relatively late in most chronic CNS diseases. This delay can be attributed to robust resilience and protection systems inherent to the nervous system. Intrinsic neuronal resilience includes, for example, the proteostasis network encompassing an extensive machinery of molecular chaperones, enzymes, and proteases. However, neurons cannot maintain their function without supporting cells, such as astrocytes or oligodendrocytes, which sense neuronal stress and, in response, provide additional extrinsic levels of protection (see “Circuit Recovery and Repair”). Once these systems reach their limits, alternative defenses engage, resulting in graded tissue inflammation, typically starting with microglia. While such responses can be protective and restore homeostasis, when this first line of glial protection is overwhelmed by persisting noxious stimuli, inflammation can progress to the recruitment of peripheral immune cells. Under such chronic conditions, self-reinforcing inflammatory cycles can result in tissue destruction. Within SyNergy, we have expanded the perspective of resilience and protection from a neuron-centric view to one of a multicellular homeostatic network of immune cells, glia, and vasculature.
Moving forward, our major aim is to understand the molecular and cellular underpinnings of the various levels of resilience and protection in degenerative, inflammatory, and vascular CNS diseases. Our primary objective is to comprehend how these systems work together to counteract pathology and investigate how maladaptive responses can reinforce pathological processes. We believe that such a highly resolved understanding of the endogenous mechanisms of cellular resilience and protection holds the key to developing strategies that harness these mechanisms for therapeutic benefit.

Tandem Projects – Cell Resilience & Protection
- CNS protection by control of disease-associated glial states
Ninkovic, Simons - Epigenetic regulation of microglial responses to aging and inflammation
Neher, Schäfer - Inflammatory axon degeneration as a consequence of metabolic vulnerability
Kerschensteiner, Misgeld
Busse / Liesz / Gjorgjieva / Götz / Cappello / Ninkovic
Circuit Recovery & Repair is induced across CNS conditions in response to neuronal damage. This response depends on the orchestrated interplay of neurons, glia, and immune cells. Among glial repair mechanisms, remyelination, which is required for effective circuit function, plays a particularly prominent role in neuroinflammatory conditions characterized by extensive myelin loss. However, myelin repair also emerges as a therapeutic target following acute CNS injuries. Among neuronal repair mechanisms, remodeling of injured neuronal circuits, even without long-range axonal regeneration, is essential for endogenous recovery of function, albeit often insufficient. Importantly, such repair mechanisms are often limited and even more so in neurodegenerative disorders due to extensive neuronal loss. However, even in these conditions, the emergence of early biomarkers, as established by SyNergy researchers and others, can potentially expand the window of opportunity to rescue neurons before death and promote circuit rewiring and adaptation. To leverage circuit remodeling therapeutically across neurological conditions, the following two points of intervention have emerged: First, targeting mechanisms that limit the remodeling of preserved neuronal circuitry. Here, our work defined critical neuron-intrinsic, as well as extrinsic—i.e., glial and immune—regulators that affect neuronal responses at the lesion site and beyond. However, depending on lesion size or disease progression, the loss of neurons may be too extensive for functional restoration solely by circuit plasticity. To tackle this, a second intervention point is the replacement of neurons. We could show that newly generated neurons can structurally and functionally integrate into damaged existing circuitry.
Moving forward, SyNergy’s major aim is to transition our intervention strategies towards therapeutic support of circuit recovery and repair. Here, a critical step is understanding how therapeutic concepts need to be tailored to the distinct tissue environments and disease trajectories induced by degenerative, vascular, or inflammatory CNS insults. Another important step is incorporating human model systems to identify regulatory principles and modulation strategies that are conserved across species.

Tandem Projects – Circuit Recovery and Repair
- Tailored approaches to improve circuit rewiring across CNS pathologies
Bareyre, Wahl - Role of tubular ER extensions in oligodendrocytes during remyelination
Harbauer, Simons - Analysis of remyelination factors across species and injury conditions
Hemmer, Misgeld
Bareyre / Ertürk / Paquet / Neher / Dichgans
Brain Borders & Interfaces such as the blood-brain barrier and the coverings of the CNS, including meninges, ependyma, and choroid plexus, but also the bone marrow of calvaria and other bones, prove to be important participants in neurological diseases. Blood-brain barrier breakdown, often in combination with innate immune activation, critically contributes to neuroinflammation, stroke, and neurodegeneration, including Alzheimer's disease. Indeed, some mechanisms, such as fibrin-induced inflammation and oxidative stress, overlap in neuroinflammation and neurodegeneration, offering opportunities for cross-disease interventions. At the same time, the functional and temporo-spatial characteristics of humoral alterations (e.g., leakiness and immune mediators) and cellular responses (e.g., immune cell invasion and fibrosis) at the brain’s interfaces vary between conditions. Emerging technologies now enable uncovering the contribution of CNS borders and interfaces to neurological conditions with unprecedented resolution. Examples of such technologies include single-cell and spatial transcriptomics, optical tissue clearing, novel PET tracers, human iPSC-based 3D models of the neurovascular unit, and compartment-resolved proteomics. Over the last funding period, SyNergy researchers have pioneered the application of these techniques to study how distinct CNS border compartments respond to neurodegenerative, neuroinflammatory, and neurovascular insults. These studies have identified disease-crossing response patterns, including the reorganization of the extracellular matrix (ECM), as active contributors to CNS pathology. For the future, our major aim is to understand the common regulatory principles that govern the role of CNS border structures in diseases of the nervous system. We believe that resolving the temporal, spatial, and disease-specific factors that influence the cellular and molecular communication at CNS border structures and their interactions with peripheral compartments will significantly enhance our understanding of the emergence and perpetuation of CNS pathologies. This will be crucial for developing therapeutic strategies to either directly target CNS border function or exploit these interfaces for optimized CNS access.

Tandem Projects – Brain Borders & Interfaces
- Comparative analysis of marrow-derived immune cells across brain diseases
Ertürk, Liesz - Astrocyte mitochondrial regulation in metabolic control
García Cáceres, Perocchi - Myeloid cell entry and polarization in the inflamed and ischemic CNS
Dichgans, Kerschensteiner
“Pioneer” Tandems
We will continue our highly successful format of “Pioneer” Tandems. In addition to adding thematic and technological breadth to the Cluster, these Tandems also enable new collaborations, allow us to integrate new researchers (e.g., from the Early Excellence Academy or new recruitments), or explore new topics that have disease-spanning potential.
- Cell type-specific exosomes in neuro-inflammatory & -degenerative diseases
Höglinger, Cappello - Human organoids to investigate the role of MEIS transcription factors
Cappello, Winkelmann - Engineered human organoid grafts to improve tissue transplantation after stroke
Schäfer, Wahl - Oxidized dopamine as mediator of neuro-immune interactions in Parkinson' disease
Burbulla, Zhou
Our Technology Hubs for methodological support:
The Hubs provide access for SyNergy members to two arcs of ‘omics’- and imaging-oriented technologies across different levels and scales of analysis from systems to clinics.
The Hubs are organized as institutions-spanning units – all Hubs are coordinated by three SyNergy experts from at least two different institutions.
Open Science
SyNergy has expressed its commitment to promoting research transparency and reproducibility in both quantitative and qualitative research endeavors. We view the ability to replicate and reproduce quantitative or qualitative research findings as fundamental pillars supporting the generalizability and reliability of scientific knowledge.
SyNergy is a member of the Open Science Center.
Transition Topics
This new measure is based on our view that pursuing the transition of a potential translational target requires larger and multidisciplinary teams of researchers including clinician-scientist expertise. Transition Topics are more complex and structurally more scalable than Tandems to match the needs of a specific scientific challenge. Four to six SyNergy Investigators explore as a team medical potential of a disease-spanning pathomechanism or advancing a target along the transition steps towards clinical application by pursuing multi-aim workpackages.
Tandem Projects are highly collaborative research projects.The projects combine expertise across traditional pathomechanisms, as well as systems biology and systems neuroscience tools. Furthermore, in many projects, the research efforts of scientists and clinicians are bundled. This allows us to combine approaches that range from in vitro models to investigator-initiated trials.
“Pioneer” Tandems: In addition to adding thematic and technological breadth to the Cluster, these Tandems also enable new collaborations, allow us to integrate new researchers (e.g., from the Early Excellence Academy or new recruitments), or explore new topics that have disease-spanning potential as the portfolio of projects within the cluster is dynamic.